Abstract
The treatment landscape of acute myeloid leukemia (AML) is evolving with new targets entering clinical translation. However, patients’ responses are still heterogeneous. Bone marrow (BM) is considered an immune privilege organ, but recent evidence suggests that inflammatory insults can increase cell cycling in hematopoietic stem cells (HSCs) gradually accumulating DNA damage. Thus, discovering immune targets on leukemia cells is an urgent unmet need.
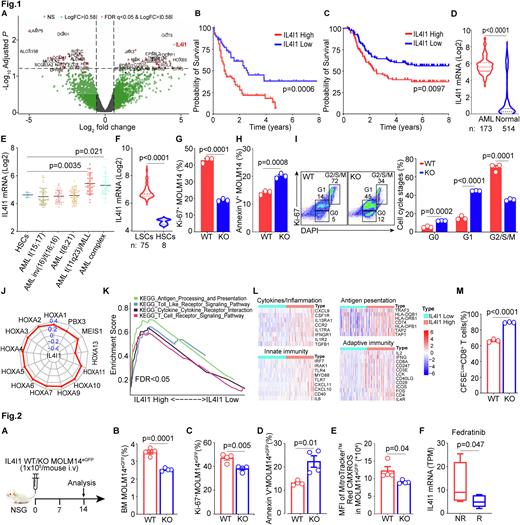
Here, we used scRNA seq to compare BM aspirates from 12 complete response (CR) and 10 refractory patients. We found 24 cell clusters expressing established markers of hematopoietic population. Differentially expressed genes between CR and refractory patients were determined using EdgeR and illustrated by volcano plots (Log2FC>|0.58|, x-axis, and Log10Adjusted P, y-axis) followed by enrichment for immune markers. Interleukin 4-induced gene 1 (IL4I1), in cluster 5 (promonocyte-like AML) was the most significant immune marker overexpressed in refractory patients (Fig. 1A). IL4I1 encodes a secreted oxidase protein (Molinier-Frenkel et al. FEBS 2017) that induces cancer immune escape by limiting proliferation of cytotoxic T cells (Lasoudris et al. Eur J Immunol. 2011). IL4I1 also activates the aryl hydrocarbon receptor and correlates with lower survival in glioma patients (Sadik et al. Cell. 2020). Its role in AML is unknown.
To determine its clinical relevance in AML, we generated Kaplan-Meier curves using TCGA (n=132) and TARGET AML (n=187) databases and showed that patients with high IL4I1 had decreased survival (Fig. 1B&C). IL4I1 expression is increased in AML patients’ samples as compared to normal blood samples (Fig. 1D) and found in patients with mixed lineage leukemia (MLL) or complex cytogenetics (Fig. 1E). We next interrogated IL4I1 expression on leukemia stem cells (LSCs) defined as CD34+CD38- in the Princess Margaret Leukemia database (n=75) and noted IL4I1 expression was higher on LSCs vs normal HSCs (Fig. 1F). We then knocked out the IL4I1 gene in MOLM14 leukemic cells using CRISPR/Cas9 technology. IL4I1 silencing inhibited leukemia proliferation measured by Ki67 (Fig. 1G) and increased leukemia apoptosis measured by Annexin-V (Fig. 1H) compared to wild type (WT) leukemic cells. IL4I1 deficiency in leukemic cells arrested G2/S/M cell cycle progression which accelerated cell differentiation as compared to WT leukemic cells (Fig. 1I). We also performed Pearson correlations of IL4I1 with leukemic oncogenic drivers such as homeobox A cluster (HOXA) genes which strongly associate with the MLL translocation (Ernst et al. Curr. Biol. 2004) in TCGA- and TARGET-AML databases. Radar plots showed that IL4I1 was significantly correlated with these HOXA family genes (Fig. 1J, TCGA shown). We next interrogated immune regulatory functions of IL4I1 in AML patients and showed that enrichment of IL4I1 high genes vs low were in 4 signaling pathways: (i) cytokines/inflammation, (ii) innate immunity, (iii) antigen presentation and (iv) adaptive immunity (Fig. 1K). Representative genes signatures in these 4 pathways are summarized in Fig. 1L. We further tested whether IL4I1 expressing leukemia cells (WT) suppress T cells expansion in vitro. We used CFSE dilution to measure CD8+ T cells proliferation. WT MOLM14egfp cells inhibited CD8+ T cell proliferation while IL4I1 blockade in MOLM14egfp cells restored their proliferation (Fig. 1M).
To test the role of IL4I1 in AML progression in vivo, we injected NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice with IL4I1-CRISPR/Cas9 KO or WT MOLM14egfp cells (Fig. 2A). Mice injected with WT MOLM14egfp cells had higher leukemia burden (Fig. 2B), higher Ki67 proliferation rate (Fig. 2C), and lower apoptosis rate (Fig. 2D) than IL4I1-KO MOLM14egfp recipients at Day 14 post-challenge. Self-renewal ability of leukemia cells is correlated to reactive oxygen species (ROS) (Testa U. et al. Exp Hematol. 2016). We found that ROS levels in IL4I1 KO leukemic cells are diminished compared to WT leukemic cells (Fig. 2E). Finally, we tested IL4I1 expression in response to Fedratinib, a JAK2 inhibitor, and showed that IL4I1 expression was decreased in responders (R) vs nonresponders (NR) (Fig. 2F).
We conclude that IL4I1 is a rational immune therapeutic target for AML and testing of IL4I1 Inhibitor CB-668 through a collaboration with Calithera is underway.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal